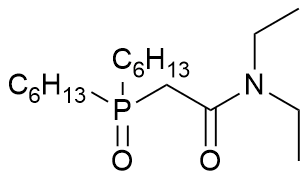
dihexyl N,N-diethylcarbamoylmethylphosphine oxide
Abbreviation(s)
DHDECMPO
Chemical group
carbamylalkylphosphine oxide
Formula
C18H38NO2P not CHON
Molecular mass
331.481 g/mol
Separation factors
| Factor | Value | Conc. | Add. ligand | Aq. acid | Organic diluent | Temp. | Contact time | Additional | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Am(III)/Fe(III) | 0.19 | 0.5 M | HNO3 0.01 M | p-diisopropylbenzene | 25°C | 1 min | [1] | ||
| Am(III)/Fe(III) | 5 | 0.5 M | HNO3 0.10 M | p-diisopropylbenzene | 25°C | 1 min | [1] | ||
| Am(III)/Fe(III) | 5.8 | 0.5 M | HNO3 0.25 M | p-diisopropylbenzene | 25°C | 1 min | [1] | ||
| Am(III)/Fe(III) | 6.1 | 0.5 M | HNO3 0.50 M | p-diisopropylbenzene | 25°C | 1 min | [1] | ||
| Am(III)/Fe(III) | 5.2 | 0.5 M | HNO3 1.00 M | p-diisopropylbenzene | 25°C | 1 min | [1] | ||
| Am(III)/Fe(III) | 1.8 | 0.5 M | HNO3 2.00 M | p-diisopropylbenzene | 25°C | 1 min | [1] | ||
| Am(III)/Fe(III) | 0.1 | 0.5 M | HNO3 4.00 M | p-diisopropylbenzene | 25°C | 1 min | [1] | ||
| Am(III)/Fe(III) | 0.0099 | 0.5 M | HNO3 6.00 M | p-diisopropylbenzene | 25°C | 1 min | [1] | ||
| Am(III)/Eu(III) | 1.06 | 0.5 M | HNO3 0.10 M | xylene | 25°C | 1 min | [2] | ||
| Am(III)/Eu(III) | 1.02 | 0.5 M | HNO3 0.25 M | xylene | 25°C | 1 min | [2] | ||
| Am(III)/Eu(III) | 1.01 | 0.5 M | HNO3 0.50 M | xylene | 25°C | 1 min | [2] | ||
| Am(III)/Eu(III) | 1.12 | 0.5 M | HNO3 1.0 M | xylene | 25°C | 1 min | [2] | ||
| Am(III)/Eu(III) | 1.25 | 0.5 M | HNO3 2.0 M | xylene | 25°C | 1 min | [2] | ||
| Am(III)/Eu(III) | 1.32 | 0.5 M | HNO3 3.0 M | xylene | 25°C | 1 min | [2] | ||
| Am(III)/Eu(III) | 1.28 | 0.5 M | HNO3 4.0 M | xylene | 25°C | 1 min | [2] | ||
| Am(III)/Eu(III) | 1.05 | 0.5 M | HNO3 6.0 M | xylene | 25°C | 1 min | [2] | ||
| Am/Fe | 12 | 0.25 M | HNO3 1 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Y | 2.9 | 0.25 M | HNO3 1 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Zr | 2.2 | 0.25 M | HNO3 1 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Mo | 0.59 | 0.25 M | HNO3 1 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Tc | 4.7 | 0.25 M | HNO3 1 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Ru | 18 | 0.25 M | HNO3 1 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Rh | 750 | 0.25 M | HNO3 1 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Pd | 4 | 0.25 M | HNO3 1 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/La | 2.5 | 0.25 M | HNO3 1 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Ce | 1.5 | 0.25 M | HNO3 1 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Pr | 1 | 0.25 M | HNO3 1 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Nd | 1.2 | 0.25 M | HNO3 1 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Sm | 1.1 | 0.25 M | HNO3 1 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Eu | 0.95 | 0.25 M | HNO3 1 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Fe | 0.49 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Y | 1.8 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Zr | 0.02 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Mo | 0.08 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Tc | 6.4 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Ru | 69 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Rh | 130 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Pd | 4.6 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/La | 3 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Ce | 1.6 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Pr | 1.3 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Nd | 1.2 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Sm | 1 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Eu | 1.1 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Fe | 0.05 | 0.25 M | HNO3 6 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Y | 0.98 | 0.25 M | HNO3 6 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Zr | 0.25 | 0.25 M | HNO3 6 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Mo | 0.3 | 0.25 M | HNO3 6 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Tc | 15 | 0.25 M | HNO3 6 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Ru | 5.3 | 0.25 M | HNO3 6 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Rh | 110 | 0.25 M | HNO3 6 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Pd | 9.7 | 0.25 M | HNO3 6 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/La | 2.8 | 0.25 M | HNO3 6 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Ce | 1.4 | 0.25 M | HNO3 6 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Pr | 1.1 | 0.25 M | HNO3 6 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Nd | 1.1 | 0.25 M | HNO3 6 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Sm | 0.84 | 0.25 M | HNO3 6 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am/Eu | 0.85 | 0.25 M | HNO3 6 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Sc/La | 128 | 0.24 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] |
Distribution coefficients
| Element | Value | Conc. | Add. ligand | Aq. acid | Organic diluent | Temp. | Contact time | Additional | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Am(III) | 0.419 | 0.5 M | HNO3 0.01 M | p-diisopropylbenzene | 25°C | 1 min | [3] | ||
| Am(III) | 27.7 | 0.5 M | HNO3 0.10 M | p-diisopropylbenzene | 25°C | 1 min | [3] | ||
| Am(III) | 80.7 | 0.5 M | HNO3 0.25 M | p-diisopropylbenzene | 25°C | 1 min | [3] | ||
| Am(III) | 105 | 0.5 M | HNO3 0.50 M | p-diisopropylbenzene | 25°C | 1 min | [3] | ||
| Am(III) | 76.4 | 0.5 M | HNO3 1.00 M | p-diisopropylbenzene | 25°C | 1 min | [3] | ||
| Am(III) | 33.2 | 0.5 M | HNO3 2.00 M | p-diisopropylbenzene | 25°C | 1 min | [3] | ||
| Am(III) | 17.5 | 0.5 M | HNO3 4.00 M | p-diisopropylbenzene | 25°C | 1 min | [3] | ||
| Am(III) | 15.5 | 0.5 M | HNO3 6.00 M | p-diisopropylbenzene | 25°C | 1 min | [3] | ||
| Fe(III) | 2.16 | 0.5 M | HNO3 0.01 M | p-diisopropylbenzene | 25°C | 1 min | [3] | ||
| Fe(III) | 5.54 | 0.5 M | HNO3 0.10 M | p-diisopropylbenzene | 25°C | 1 min | [3] | ||
| Fe(III) | 13.9 | 0.5 M | HNO3 0.25 M | p-diisopropylbenzene | 25°C | 1 min | [3] | ||
| Fe(III) | 17.3 | 0.5 M | HNO3 0.50 M | p-diisopropylbenzene | 25°C | 1 min | [3] | ||
| Fe(III) | 14.7 | 0.5 M | HNO3 1.00 M | p-diisopropylbenzene | 25°C | 1 min | [3] | ||
| Fe(III) | 18 | 0.5 M | HNO3 2.00 M | p-diisopropylbenzene | 25°C | 1 min | [3] | ||
| Fe(III) | 175 | 0.5 M | HNO3 4.00 M | p-diisopropylbenzene | 25°C | 1 min | [3] | ||
| Fe(III) | 1570 | 0.5 M | HNO3 6.00 M | p-diisopropylbenzene | 25°C | 1 min | [3] | ||
| Am(III) | ≈25 | 0.25 M | HNO3 0.25 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am(III) | ≈30 | 0.25 M | HNO3 0.5 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am(III) | ≈18 | 0.25 M | HNO3 1 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am(III) | ≈2.2 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am(III) | ≈1.5 | 0.25 M | HNO3 6 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Fe | 4.5 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Y | 1.2 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Zr | 110 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Mo | 28 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Tc | 0.34 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Ru | 0.32 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Rh | 0.02 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Pd | 0.48 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| La | 0.73 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Ce | 1.4 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Pr | 1.7 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Nd | 1.8 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Sm | 2.2 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Eu | 2 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] | ||
| Am | 2.2 | 0.25 M | HNO3 3 M | Tetrachloroethylene | 25°C | 1 min | [3] |