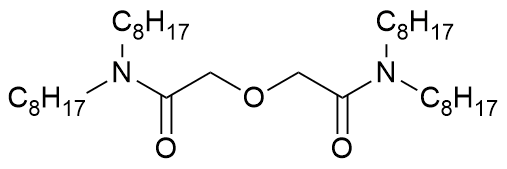
N,N,N',N'-tetraoctyl diglycolic amide
Abbreviation(s)
TODGA, TOOPDA
Chemical group
diglycolamide
Formula
C36H72N2O3 CHON
Molecular mass
580.983 g/mol
Processes
Elements separated
An(III), Ln(III), An(IV)
Country of origin
![]() Japan
Japan
Country that has performed tests
![]() Japan,
Japan,
![]() USA,
USA,
![]() Russia,
Russia,
![]() Sweden,
Sweden,
![]() UK,
UK,
![]() Germany
Germany
Separation factors
| Factor | Value | Conc. | Add. ligand | Aq. acid | Organic diluent | Temp. | Contact time | Additional | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| La/Am | ≈0.94 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.3 pH | [1] | |
| Ce/Am | ≈1.30 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.3 pH | [1] | |
| Pr/Am | ≈1.80 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.3 pH | [1] | |
| Nd/Am | ≈2.14 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.3 pH | [1] | |
| Sm/Am | ≈3.91 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.3 pH | [1] | |
| Eu/Am | ≈5.10 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.3 pH | [1] | |
| Gd/Am | ≈8.64 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.3 pH | [1] | |
| La/Am | ≈2.22 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.4 pH | [1] | |
| Ce/Am | ≈3.72 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.4 pH | [1] | |
| Pr/Am | ≈3.96 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.4 pH | [1] | |
| Nd/Am | ≈4.12 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.4 pH | [1] | |
| Sm/Am | ≈4.07 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.4 pH | [1] | |
| Eu/Am | ≈5.16 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.4 pH | [1] | |
| Gd/Am | ≈8.11 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.4 pH | [1] | |
| La/Am | ≈5.16 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.5 pH | [1] | |
| Ce/Am | ≈10.30 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.5 pH | [1] | |
| Pr/Am | ≈7.06 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.5 pH | [1] | |
| Nd/Am | ≈5.36 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.5 pH | [1] | |
| Sm/Am | ≈4.38 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.5 pH | [1] | |
| Eu/Am | ≈5.43 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.5 pH | [1] | |
| Gd/Am | ≈7.15 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.5 pH | [1] | |
| La/Am | ≈12.43 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.6 pH | [1] | |
| Ce/Am | ≈14.10 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.6 pH | [1] | |
| Pr/Am | ≈9.20 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.6 pH | [1] | |
| Nd/Am | ≈7.06 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.6 pH | [1] | |
| Sm/Am | ≈4.55 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.6 pH | [1] | |
| Eu/Am | ≈5.56 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.6 pH | [1] | |
| Gd/Am | ≈7.06 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.6 pH | [1] | |
| La/Am | ≈27.43 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [1] | |
| Ce/Am | ≈22.72 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [1] | |
| Pr/Am | ≈11.53 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [1] | |
| Nd/Am | ≈9.20 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [1] | |
| Sm/Am | ≈4.79 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [1] | |
| Eu/Am | ≈5.78 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [1] | |
| Gd/Am | ≈6.23 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [1] | |
| La/Am | ≈62.05 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [1] | |
| Ce/Am | ≈40.48 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [1] | |
| Pr/Am | ≈15.98 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [1] | |
| Nd/Am | ≈12.43 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [1] | |
| Sm/Am | ≈5.03 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [1] | |
| Eu/Am | ≈6.08 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [1] | |
| Gd/Am | ≈5.78 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [1] | |
| La/Cm | ≈0.85 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.3 pH | [1] | |
| Ce/Cm | ≈1.16 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.3 pH | [1] | |
| Pr/Cm | ≈1.63 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.3 pH | [1] | |
| Nd/Cm | ≈1.92 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.3 pH | [1] | |
| Sm/Cm | ≈8.71 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.3 pH | [1] | |
| Eu/Cm | ≈11.33 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.3 pH | [1] | |
| Gd/Cm | ≈19.66 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.3 pH | [1] | |
| La/Cm | ≈1.89 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.4 pH | [1] | |
| Ce/Cm | ≈3.05 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.4 pH | [1] | |
| Pr/Cm | ≈3.32 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.4 pH | [1] | |
| Nd/Cm | ≈3.41 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.4 pH | [1] | |
| Sm/Cm | ≈10.00 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.4 pH | [1] | |
| Eu/Cm | ≈12.68 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.4 pH | [1] | |
| Gd/Cm | ≈19.66 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.4 pH | [1] | |
| La/Cm | ≈4.22 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.5 pH | [1] | |
| Ce/Cm | ≈8.50 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.5 pH | [1] | |
| Pr/Cm | ≈5.77 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.5 pH | [1] | |
| Nd/Cm | ≈4.55 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.5 pH | [1] | |
| Sm/Cm | ≈11.19 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.5 pH | [1] | |
| Eu/Cm | ≈14.02 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.5 pH | [1] | |
| Gd/Cm | ≈18.93 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.5 pH | [1] | |
| La/Cm | ≈9.63 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.6 pH | [1] | |
| Ce/Cm | ≈10.92 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.6 pH | [1] | |
| Pr/Cm | ≈7.22 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.6 pH | [1] | |
| Nd/Cm | ≈5.48 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.6 pH | [1] | |
| Sm/Cm | ≈13.01 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.6 pH | [1] | |
| Eu/Cm | ≈15.50 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.6 pH | [1] | |
| Gd/Cm | ≈19.66 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.6 pH | [1] | |
| La/Cm | ≈20.15 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [1] | |
| Ce/Cm | ≈16.91 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [1] | |
| Pr/Cm | ≈8.29 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [1] | |
| Nd/Cm | ≈6.70 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [1] | |
| Sm/Cm | ≈14.74 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [1] | |
| Eu/Cm | ≈17.78 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [1] | |
| Gd/Cm | ≈19.90 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [1] | |
| La/Cm | ≈44.89 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [1] | |
| Ce/Cm | ≈30.08 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [1] | |
| Pr/Cm | ≈11.77 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [1] | |
| Nd/Cm | ≈9.28 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [1] | |
| Sm/Cm | ≈17.13 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [1] | |
| Eu/Cm | ≈19.66 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [1] | |
| Gd/Cm | ≈19.17 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [1] | |
| Ca/Sr | 15 | HNO3 2.9 M | n-Dodecane | 25°C | 120 min | average SF; 0.005-0.1M TODGA | [2] | ||
| Sr/Ba | 78 | HNO3 2.9 M | n-Dodecane | 25°C | 120 min | average SF; 0.02-0.5M TODGA | [2] | ||
| Ca/Sr | 39 | 0.1 M | HNO3 4.65 M | n-Dodecane | 25°C | 120 min | [2] | ||
| Sr/Ba | 197 | 0.1 M | HNO3 4.65 M | n-Dodecane | 25°C | 120 min | [2] | ||
| Am/La | 4.8 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 20 min | [3] | ||
| Nd/Am | 1.47 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 20 min | [3] | ||
| Cm/Am | 1.89 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 20 min | [3] | ||
| Gd/La | 33.2 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 20 min | [3] | ||
| Eu/Am | 0.01 | 0.2 M | HNO3 0.09 M | hydrogenated tetrapropene/1-Octanol, 5% | 20°C | 15 min | [4] | ||
| Eu/Am | ≈0.01 | 0.2 M | HNO3 0.19 M | hydrogenated tetrapropene/1-Octanol, 5% | 20°C | 15 min | [4] | ||
| Eu/Am | ≈0.005 | 0.2 M | HNO3 0.45 M | hydrogenated tetrapropene/1-Octanol, 5% | 20°C | 15 min | [4] | ||
| Cm(III)/Am(III) | ≈1.6 | 0.2 M | HNO3 0.5 M | hydrogenated tetrapropene/1-Octanol, 5% | 20°C | 15 min | [4] | ||
| Eu(III)/Am(III) | ≈7.5 | 0.2 M | HEDTA 0.02 M | HNO3 4.2 M | hydrogenated tetrapropene/1-Octanol, 5% | 20°C | 15 min | 0.2M Oxalic Acid | [4] |
| La(III)/Am(III) | 0.18 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [5] | ||
| Eu(III)/Am(III) | 8.8 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [5] | ||
| Lu(III)/Am(III) | 21 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [5] | ||
| Th(IV)/Am(III) | 4.9 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [5] | ||
| U(VI)/Am(III) | 0.027 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [5] | ||
| Np(V)/Am(III) | 0.0002 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [5] | ||
| Am(III)/Am(III) | 1 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [5] | ||
| Cm(III)/Am(III) | 2.6 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [5] | ||
| Cf(III)/Am(III) | 5.2 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [5] | ||
| Eu(III)/Am(III) | 1.9 | 0.1 M | HNO3 3 M | HPT/1-Octanol, 1:1 | 22°C | 10 min | [6] |
Distribution coefficients
| Element | Value | Conc. | Add. ligand | Aq. acid | Organic diluent | Temp. | Contact time | Additional | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Ce | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [7] | |
| Cs | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [7] | |
| Rh | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [7] | |
| Te | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [7] | |
| Al | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [7] | |
| Na | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [7] | |
| Zr | ≤0.001 | 0.2 M | TBP 0.5 M | HNO3 1 M | hydrogenated tetrapropene | 22°C | 15 min | 0.025M CDTA; 0.0004M Zr | [7] |
| Zr | ≤0.001 | 0.2 M | TBP 0.5 M | HNO3 1 M | hydrogenated tetrapropene | 22°C | 15 min | 0.025M CDTA; 0.001M Zr | [7] |
| Zr | ≤0.001 | 0.2 M | TBP 0.5 M | HNO3 1 M | hydrogenated tetrapropene | 22°C | 15 min | 0.025M CDTA; 0.00015M Zr | [7] |
| Zr | 0.002 | 0.2 M | TBP 0.5 M | HNO3 1 M | hydrogenated tetrapropene | 22°C | 15 min | 0.025M CDTA; 0.003M Zr | [7] |
| Zr | 0.004 | 0.2 M | TBP 0.5 M | HNO3 1 M | hydrogenated tetrapropene | 22°C | 15 min | 0.025M CDTA; 0.006M Zr | [7] |
| Zr | 0.011 | 0.2 M | TBP 0.5 M | HNO3 1 M | hydrogenated tetrapropene | 22°C | 15 min | 0.025M CDTA; 0.012M Zr | [7] |
| Zr | ≤0.001 | 0.2 M | TBP 0.5 M | HNO3 3 M | hydrogenated tetrapropene | 22°C | 15 min | 0.05M CDTA; 0.0004M Zr | [7] |
| Zr | ≤0.001 | 0.2 M | TBP 0.5 M | HNO3 3 M | hydrogenated tetrapropene | 22°C | 15 min | 0.05M CDTA; 0.001M Zr | [7] |
| Zr | ≤0.001 | 0.2 M | TBP 0.5 M | HNO3 3 M | hydrogenated tetrapropene | 22°C | 15 min | 0.05M CDTA; 0.00015M Zr | [7] |
| Zr | ≤0.001 | 0.2 M | TBP 0.5 M | HNO3 3 M | hydrogenated tetrapropene | 22°C | 15 min | 0.05M CDTA; 0.003M Zr | [7] |
| Zr | ≤0.001 | 0.2 M | TBP 0.5 M | HNO3 3 M | hydrogenated tetrapropene | 22°C | 15 min | 0.05M CDTA; 0.006M Zr | [7] |
| Zr | 0.005 | 0.2 M | TBP 0.5 M | HNO3 3 M | hydrogenated tetrapropene | 22°C | 15 min | 0.05M CDTA; 0.012M Zr | [7] |
| Zr(IV) | 350 | 0.1 M | HNO3 3 M | hydrogenated tetrapropene/octanol, 95:5 | 22°C | 15 min | [8] | ||
| Pd(II) | 20 | 0.1 M | HNO3 3 M | hydrogenated tetrapropene/octanol, 95:5 | 22°C | 15 min | [8] | ||
| Sr(II) | 3.2 | 0.1 M | HNO3 3 M | hydrogenated tetrapropene/octanol, 95:5 | 22°C | 15 min | [8] | ||
| Mo(IV) | 0.23 | 0.1 M | HNO3 3 M | hydrogenated tetrapropene/octanol, 95:5 | 22°C | 15 min | [8] | ||
| Zr(IV) | 1.43 | 0.1 M | HEDTA 0.05 M | HNO3 3 M | hydrogenated tetrapropene/octanol, 95:5 | 22°C | 15 min | 0.2M oxalic acid | [8] |
| Pd(II) | 0.4 | 0.1 M | HEDTA 0.05 M | HNO3 3 M | hydrogenated tetrapropene/octanol, 95:5 | 22°C | 15 min | 0.2M oxalic acid | [8] |
| Sr(II) | 2.88 | 0.1 M | HEDTA 0.05 M | HNO3 3 M | hydrogenated tetrapropene/octanol, 95:5 | 22°C | 15 min | 0.2M oxalic acid | [8] |
| Mo(IV) | 0.07 | 0.1 M | HEDTA 0.05 M | HNO3 3 M | hydrogenated tetrapropene/octanol, 95:5 | 22°C | 15 min | 0.2M oxalic acid | [8] |
| Am(III) | 24 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 45 min | SHLW | [9] | |
| Am(III) | 0.3 | 0.1 M | HNO3 1 M | toluene | 25°C | 45 min | SHLW | [9] | |
| Am(III) | 68 | 0.1 M | HNO3 1 M | 1-octanol | 25°C | 45 min | SHLW | [9] | |
| Am(III) | 12 | 0.1 M | HNO3 1 M | 1,2-dichloroethane | 25°C | 45 min | SHLW | [9] | |
| Am(III) | 202 | 0.1 M | HNO3 1 M | nitrobenzene | 25°C | 45 min | SHLW | [9] | |
| Am | 0.001 | 0.1 M | HNO3 0.01 M | n-Dodecane | 25°C | 45 min | SHLW | [9] | |
| Am | 0.013 | 0.1 M | HNO3 0.1 M | n-Dodecane | 25°C | 45 min | SHLW | [9] | |
| Am | 0.78 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 45 min | SHLW | [9] | |
| Am | 24 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 45 min | SHLW | [9] | |
| Am | 171 | 0.1 M | HNO3 2 M | n-Dodecane | 25°C | 45 min | SHLW | [9] | |
| Am | 297 | 0.1 M | HNO3 3 M | n-Dodecane | 25°C | 45 min | SHLW | [9] | |
| Am | 349 | 0.1 M | HNO3 4 M | n-Dodecane | 25°C | 45 min | SHLW | [9] | |
| Am | 336 | 0.1 M | HNO3 5 M | n-Dodecane | 25°C | 45 min | SHLW | [9] | |
| Am | 341 | 0.1 M | HNO3 6 M | n-Dodecane | 25°C | 45 min | SHLW | [9] | |
| Am | 0.001 | 0.1 M | DHOA 0.5 M | HNO3 0.01 M | n-Dodecane | 25°C | 45 min | SHLW | [9] |
| Am | 0.006 | 0.1 M | DHOA 0.5 M | HNO3 0.1 M | n-Dodecane | 25°C | 45 min | SHLW | [9] |
| Am | 0.24 | 0.1 M | DHOA 0.5 M | HNO3 0.5 M | n-Dodecane | 25°C | 45 min | SHLW | [9] |
| Am | 9.8 | 0.1 M | DHOA 0.5 M | HNO3 1 M | n-Dodecane | 25°C | 45 min | SHLW | [9] |
| Am | 176 | 0.1 M | DHOA 0.5 M | HNO3 2 M | n-Dodecane | 25°C | 45 min | SHLW | [9] |
| Am | 274 | 0.1 M | DHOA 0.5 M | HNO3 3 M | n-Dodecane | 25°C | 45 min | SHLW | [9] |
| Am | 289 | 0.1 M | DHOA 0.5 M | HNO3 4 M | n-Dodecane | 25°C | 45 min | SHLW | [9] |
| Am | 284 | 0.1 M | DHOA 0.5 M | HNO3 5 M | n-Dodecane | 25°C | 45 min | SHLW | [9] |
| Am | 319 | 0.1 M | DHOA 0.5 M | HNO3 6 M | n-Dodecane | 25°C | 45 min | SHLW | [9] |
| Y | >100 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| Ce | >100 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| Pr | >100 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| Nd | >100 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| Sm | >100 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| La | >100 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| Eu | 68 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| Gd | >100 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| Mo | 1.7 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| Sr | 2 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| Pd | 0.22 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| Se | 0.18 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| Cd | 0.16 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| Ru | 0.41 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| Ba | 0.14 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| Cs | <0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| Zr | <0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| Ag | <0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| Ni | <0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| Rb | <0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| Rh | <0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| Sn | <0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| Sb | <0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| Te | <0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| Cu | <0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3.2 M | Exxsol-D80 | 22°C | 15 min | simulated HAR; 0.05M CDTA | [10] |
| Eu(III) | ≈300 | 0.2 M | HNO3 | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | 0.5M NH4NO3 | [10] | |
| Am(III) | ≈60 | 0.2 M | HNO3 | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | 0.5M NH4NO3 | [10] | |
| Eu(III) | 950 | 0.2 M | HNO3 0.5 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [10] | ||
| Am(III) | 120 | 0.2 M | HNO3 0.5 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [10] | ||
| Y(III) | ≈200 | 0.2 M | HNO3 0.15 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [10] | ||
| Y(III) | ≈500 | 0.2 M | HNO3 0.25 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [10] | ||
| Y(III) | ≈900 | 0.2 M | HNO3 0.35 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [10] | ||
| La | ≈0.47 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 0.97 pH | [10] | ||
| Ce | ≈0.71 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 0.97 pH | [10] | ||
| Pr | ≈0.75 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 0.97 pH | [10] | ||
| Nd | ≈0.84 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 0.97 pH | [10] | ||
| Sm | ≈3.15 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 0.97 pH | [10] | ||
| Eu | ≈5.93 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 0.97 pH | [10] | ||
| Gd | ≈8.48 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 0.97 pH | [10] | ||
| Tb | ≈17.99 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 0.97 pH | [10] | ||
| Dy | ≈28.46 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 0.97 pH | [10] | ||
| Ho | ≈38.52 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 0.97 pH | [10] | ||
| Er | ≈44.61 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 0.97 pH | [10] | ||
| Tm | ≈46.27 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 0.97 pH | [10] | ||
| Yb | ≈50.26 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 0.97 pH | [10] | ||
| Lu | ≈52.62 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 0.97 pH | [10] | ||
| La | ≈0.36 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.55 pH | [10] | ||
| Ce | ≈1.00 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.55 pH | [10] | ||
| Pr | ≈0.58 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.55 pH | [10] | ||
| Nd | ≈0.76 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.55 pH | [10] | ||
| Sm | ≈1.95 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.55 pH | [10] | ||
| Eu | ≈3.68 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.55 pH | [10] | ||
| Gd | ≈5.17 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.55 pH | [10] | ||
| Tb | ≈11.48 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.55 pH | [10] | ||
| Dy | ≈17.82 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.55 pH | [10] | ||
| Ho | ≈25.73 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.55 pH | [10] | ||
| Er | ≈31.19 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.55 pH | [10] | ||
| Tm | ≈31.77 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.55 pH | [10] | ||
| Yb | ≈35.14 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.55 pH | [10] | ||
| Lu | ≈37.13 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.55 pH | [10] | ||
| La | ≈0.35 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 2.03 pH | [10] | ||
| Ce | ≈1.26 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 2.03 pH | [10] | ||
| Pr | ≈0.56 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 2.03 pH | [10] | ||
| Nd | ≈0.79 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 2.03 pH | [10] | ||
| Sm | ≈1.83 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 2.03 pH | [10] | ||
| Eu | ≈3.33 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 2.03 pH | [10] | ||
| Gd | ≈4.71 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 2.03 pH | [10] | ||
| Tb | ≈10.09 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 2.03 pH | [10] | ||
| Dy | ≈16.72 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 2.03 pH | [10] | ||
| Ho | ≈23.04 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 2.03 pH | [10] | ||
| Er | ≈27.68 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 2.03 pH | [10] | ||
| Tm | ≈29.79 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 2.03 pH | [10] | ||
| Yb | ≈32.06 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 2.03 pH | [10] | ||
| Lu | ≈34.82 | 0.1 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 2.03 pH | [10] | ||
| La | ≈0.59 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.25 pH | [10] | |
| Ce | ≈0.94 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.25 pH | [10] | |
| Pr | ≈1.22 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.25 pH | [10] | |
| Nd | ≈1.32 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.25 pH | [10] | |
| Sm | ≈6.11 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.25 pH | [10] | |
| Eu | ≈14.07 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.25 pH | [10] | |
| Gd | ≈29.45 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.25 pH | [10] | |
| Tb | ≈34.93 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.25 pH | [10] | |
| Dy | ≈59.38 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.25 pH | [10] | |
| Ho | ≈97.20 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.25 pH | [10] | |
| Er | ≈97.20 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.25 pH | [10] | |
| Tm | ≈115.27 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.25 pH | [10] | |
| Yb | ≈119.73 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.25 pH | [10] | |
| Lu | ≈122.02 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.25 pH | [10] | |
| La | ≈0.47 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.42 pH | [10] | |
| Ce | ≈0.77 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.42 pH | [10] | |
| Pr | ≈0.85 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.42 pH | [10] | |
| Nd | ≈0.88 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.42 pH | [10] | |
| Sm | ≈1.56 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.42 pH | [10] | |
| Eu | ≈2.24 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.42 pH | [10] | |
| Gd | ≈3.40 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.42 pH | [10] | |
| Tb | ≈5.15 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.42 pH | [10] | |
| Dy | ≈8.12 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.42 pH | [10] | |
| Ho | ≈14.89 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.42 pH | [10] | |
| Er | ≈73.15 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.42 pH | [10] | |
| Tm | ≈93.58 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.42 pH | [10] | |
| Yb | ≈97.20 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.42 pH | [10] | |
| Lu | ≈100.95 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.42 pH | [10] | |
| La | ≈0.41 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [10] | |
| Ce | ≈0.45 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [10] | |
| Pr | ≈0.27 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [10] | |
| Nd | ≈0.25 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [10] | |
| Sm | ≈0.13 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [10] | |
| Eu | ≈0.15 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [10] | |
| Gd | ≈0.21 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [10] | |
| Tb | ≈0.24 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [10] | |
| Dy | ≈0.31 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [10] | |
| Ho | ≈0.46 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [10] | |
| Er | ≈0.65 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [10] | |
| Tm | ≈0.80 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [10] | |
| Yb | ≈1.03 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [10] | |
| Lu | ≈1.59 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.7 pH | [10] | |
| La | ≈0.24 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.9 pH | [10] | |
| Ce | ≈0.10 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.9 pH | [10] | |
| Pr | ≈0.04 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.9 pH | [10] | |
| Nd | ≈0.03 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.9 pH | [10] | |
| Sm | ≈0.01 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.9 pH | [10] | |
| Eu | ≈0.01 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.9 pH | [10] | |
| Gd | ≈0.02 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.9 pH | [10] | |
| Tb | ≈0.02 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.9 pH | [10] | |
| Dy | ≈0.02 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.9 pH | [10] | |
| Ho | ≈0.04 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.9 pH | [10] | |
| Er | ≈0.05 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.9 pH | [10] | |
| Tm | ≈0.06 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.9 pH | [10] | |
| Yb | ≈0.08 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.9 pH | [10] | |
| Lu | ≈0.11 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.9 pH | [10] | |
| La | ≈0.28 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Ce | ≈0.21 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Pr | ≈0.10 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Nd | ≈0.05 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Sm | ≈0.04 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Gd | ≈0.05 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| La | ≈0.18 | 0.1 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Ce | ≈0.11 | 0.1 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Pr | ≈0.05 | 0.1 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Eu | ≈0.01 | 0.1 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| La | ≈0.11 | 0.1 M | DTPA 0.05 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Ce | ≈0.06 | 0.1 M | DTPA 0.05 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Pr | ≈0.02 | 0.1 M | DTPA 0.05 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Nd | ≈0.02 | 0.1 M | DTPA 0.05 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Sm | ≈0.01 | 0.1 M | DTPA 0.05 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Eu | ≈0.01 | 0.1 M | DTPA 0.05 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Gd | ≈0.01 | 0.1 M | DTPA 0.05 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Tb | ≈0.06 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Er | ≈0.13 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Tm | ≈0.16 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Yb | ≈0.23 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Lu | ≈0.32 | 0.1 M | DTPA 0.01 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Tb | ≈0.02 | 0.1 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Dy | ≈0.03 | 0.1 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Ho | ≈0.04 | 0.1 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Er | ≈0.06 | 0.1 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Tm | ≈0.07 | 0.1 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Yb | ≈0.09 | 0.1 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Lu | ≈0.13 | 0.1 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Tb | ≈0.01 | 0.1 M | DTPA 0.05 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Dy | ≈0.02 | 0.1 M | DTPA 0.05 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Ho | ≈0.03 | 0.1 M | DTPA 0.05 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Er | ≈0.04 | 0.1 M | DTPA 0.05 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Tm | ≈0.05 | 0.1 M | DTPA 0.05 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Yb | ≈0.06 | 0.1 M | DTPA 0.05 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| Lu | ≈0.09 | 0.1 M | DTPA 0.05 M | n-Dodecane | 25°C | 60 min | 1M NaNO3; 1.8 pH | [10] | |
| La | ≈1.07 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.54M NaNO3; 1.8 pH | [10] | |
| Ce | ≈1.26 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.54M NaNO3; 1.8 pH | [10] | |
| Pr | ≈0.91 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.54M NaNO3; 1.8 pH | [10] | |
| Nd | ≈0.78 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.54M NaNO3; 1.8 pH | [10] | |
| Sm | ≈0.43 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.54M NaNO3; 1.8 pH | [10] | |
| Eu | ≈0.48 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.54M NaNO3; 1.8 pH | [10] | |
| Gd | ≈0.70 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.54M NaNO3; 1.8 pH | [10] | |
| La | ≈4.56 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.88M NaNO3; 1.8 pH | [10] | |
| Ce | ≈3.10 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.88M NaNO3; 1.8 pH | [10] | |
| Nd | ≈2.81 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.88M NaNO3; 1.8 pH | [10] | |
| Eu | ≈0.97 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.88M NaNO3; 1.8 pH | [10] | |
| Gd | ≈1.46 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.88M NaNO3; 1.8 pH | [10] | |
| La | ≈31.17 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1.73M NaNO3; 1.8 pH | [10] | |
| Ce | ≈25.56 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1.73M NaNO3; 1.8 pH | [10] | |
| Pr | ≈10.60 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1.73M NaNO3; 1.8 pH | [10] | |
| Nd | ≈10.08 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1.73M NaNO3; 1.8 pH | [10] | |
| Sm | ≈3.98 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1.73M NaNO3; 1.8 pH | [10] | |
| Eu | ≈4.13 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1.73M NaNO3; 1.8 pH | [10] | |
| Gd | ≈5.77 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1.73M NaNO3; 1.8 pH | [10] | |
| La | ≈0.02 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1.15M NaNO3; 1.8 pH | [10] | |
| Ce | ≈6.45 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1.15M NaNO3; 1.8 pH | [10] | |
| Pr | ≈5.23 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1.15M NaNO3; 1.8 pH | [10] | |
| Nd | ≈3.79 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1.15M NaNO3; 1.8 pH | [10] | |
| Eu | ≈1.84 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1.15M NaNO3; 1.8 pH | [10] | |
| Gd | ≈2.64 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1.15M NaNO3; 1.8 pH | [10] | |
| Tb | ≈0.80 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.54M NaNO3; 1.8 pH | [10] | |
| Dy | ≈1.07 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.54M NaNO3; 1.8 pH | [10] | |
| Ho | ≈1.55 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.54M NaNO3; 1.8 pH | [10] | |
| Er | ≈2.14 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.54M NaNO3; 1.8 pH | [10] | |
| Tm | ≈2.46 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.54M NaNO3; 1.8 pH | [10] | |
| Yb | ≈2.90 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.54M NaNO3; 1.8 pH | [10] | |
| Lu | ≈4.38 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.54M NaNO3; 1.8 pH | [10] | |
| Tb | ≈2.82 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.88M NaNO3; 1.8 pH | [10] | |
| Dy | ≈3.87 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.88M NaNO3; 1.8 pH | [10] | |
| Ho | ≈4.97 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.88M NaNO3; 1.8 pH | [10] | |
| Er | ≈5.78 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.88M NaNO3; 1.8 pH | [10] | |
| Tm | ≈6.39 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.88M NaNO3; 1.8 pH | [10] | |
| Yb | ≈7.71 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.88M NaNO3; 1.8 pH | [10] | |
| Lu | ≈8.97 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 0.88M NaNO3; 1.8 pH | [10] | |
| Tb | ≈5.93 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1.73M NaNO3; 1.8 pH | [10] | |
| Dy | ≈7.24 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1.73M NaNO3; 1.8 pH | [10] | |
| Ho | ≈8.64 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1.73M NaNO3; 1.8 pH | [10] | |
| Er | ≈9.67 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1.73M NaNO3; 1.8 pH | [10] | |
| Tm | ≈10.56 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1.73M NaNO3; 1.8 pH | [10] | |
| Yb | ≈10.96 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1.73M NaNO3; 1.8 pH | [10] | |
| Lu | ≈12.91 | 0.2 M | DTPA 0.02 M | n-Dodecane | 25°C | 60 min | 1.73M NaNO3; 1.8 pH | [10] | |
| Ln | >100 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Zr | >100 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Pd | 4.2 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Mo | 1.93 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Sr | 1.43 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Se | 0.77 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Fe | 0.25 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Cd | 0.25 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Ru | 0.23 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Ba | 0.13 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Cr | ≤0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Ni | ≤0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Rb | ≤0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Rh | ≤0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Sn | ≤0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Sb | ≤0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Te | ≤0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Cs | ≤0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Ln | >100 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Zr | ≤0.01 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Pd | 0.07 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Mo | 2.1 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Sr | 2.25 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Se | 0.74 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Fe | 0.25 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Cd | 0.17 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Ru | 0.32 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Ba | 0.2 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Cr | ≤0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Ni | ≤0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Rb | ≤0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Rh | ≤0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Sn | ≤0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Sb | ≤0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Te | ≤0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Cs | ≤0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Zr | 30 | 0.2 M | DMDOHEMA 0.5 M | HNO3 1 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Zr | ≤0.01 | 0.2 M | DMDOHEMA 0.5 M | HNO3 1 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Mo | 1.21 | 0.2 M | DMDOHEMA 0.5 M | HNO3 1 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Mo | 1.43 | 0.2 M | DMDOHEMA 0.5 M | HNO3 1 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Zr | 86 | 0.2 M | DMDOHEMA 0.5 M | HNO3 2 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Zr | ≤0.01 | 0.2 M | DMDOHEMA 0.5 M | HNO3 2 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Mo | 1.36 | 0.2 M | DMDOHEMA 0.5 M | HNO3 2 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Mo | 1.08 | 0.2 M | DMDOHEMA 0.5 M | HNO3 2 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Zr | 142 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Zr | ≤0.01 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Mo | 1.93 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Mo | 2.1 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Zr | 376 | 0.2 M | DMDOHEMA 0.5 M | HNO3 4 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Zr | 0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 4 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Mo | 3.62 | 0.2 M | DMDOHEMA 0.5 M | HNO3 4 M | Exxsol D80 | 22°C | org/aq=1 | [11] | |
| Mo | 4.12 | 0.2 M | DMDOHEMA 0.5 M | HNO3 4 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA | [11] | |
| Am(III)241 | 65 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.01M CDTA; 17 g/L Pu239 + 0.1 g/L Am241 | [11] | |
| Pu(IV)239 | 60 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.01M CDTA; 17 g/L Pu239 + 0.1 g/L Am241 | [11] | |
| Zr(IV) | 7.7 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.01M CDTA; 17 g/L Pu239 + 0.1 g/L Am241 | [11] | |
| Pd(II) | 0.5 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.01M CDTA; 17 g/L Pu239 + 0.1 g/L Am241 | [11] | |
| Am(III)241 | 42 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA; 17 g/L Pu239 + 0.1 g/L Am241 | [11] | |
| Pu(IV)239 | 35 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA; 17 g/L Pu239 + 0.1 g/L Am241 | [11] | |
| Zr(IV) | 1.2 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA; 17 g/L Pu239 + 0.1 g/L Am241 | [11] | |
| Pd(II) | 0.05 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.05M CDTA; 17 g/L Pu239 + 0.1 g/L Am241 | [11] | |
| Am(III)241 | 40 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.1M CDTA; 17 g/L Pu239 + 0.1 g/L Am241 | [11] | |
| Pu(IV)239 | 32 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.1M CDTA; 17 g/L Pu239 + 0.1 g/L Am241 | [11] | |
| Zr(IV) | 0.2 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.1M CDTA; 17 g/L Pu239 + 0.1 g/L Am241 | [11] | |
| Pd(II) | 0.02 | 0.2 M | DMDOHEMA 0.5 M | HNO3 3 M | Exxsol D80 | 22°C | org/aq=1; 0.1M CDTA; 17 g/L Pu239 + 0.1 g/L Am241 | [11] | |
| Am241 | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Pu239 | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Y | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| La | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Ce(IV) | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Pr | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Nd | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Sm | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Eu | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Eu152 | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Gd | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Zr | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Pd | 9.31 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Ag | 0.69 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Ba | 0.13 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Cd | 0.14 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Mo | 0.26 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Ni | 0.05 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Sr | 1.5 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Rb | 0.1 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Ru | 0.34 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Cr | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Cu | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Sb | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Sn | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Se | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Ce | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Cs | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Rh | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Te | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Al | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Na | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR | [11] |
| Am241 | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Pu239 | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Y | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| La | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Ce(IV) | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Pr | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Nd | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Sm | 99.2 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Eu | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Eu152 | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Gd | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Zr | 0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Pd | 0.07 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Ag | 0.06 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Ba | 0.08 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Cd | 0.08 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Mo | 0.12 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Ni | 0.02 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Sr | 2.84 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Rb | 0.03 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Ru | 0.32 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Cr | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Cu | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Sb | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Sn | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Se | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Ce | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Cs | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Rh | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Te | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Al | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Na | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M EDTA | [11] |
| Am241 | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Pu239 | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Y | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| La | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Ce(IV) | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Pr | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Nd | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Sm | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Eu | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Eu152 | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Gd | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Zr | 0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Pd | 0.07 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Ag | 0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Ba | 0.03 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Cd | 0.08 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Mo | 0.19 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Ni | 0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Sr | 3.31 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Rb | 0.14 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Ru | 0.32 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Cr | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Cu | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Sb | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Sn | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Se | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Ce | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Cs | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Rh | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Te | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Al | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Na | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M CDTA | [11] |
| Am241 | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Y | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| La | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Ce(IV) | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Pr | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Nd | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Sm | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Eu | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Eu152 | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Gd | >100 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Zr | 32.8 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Pd | 0.07 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Ag | 0.59 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Ba | 0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Cd | 0.14 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Mo | 0.23 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Ni | 0.08 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Sr | 1.54 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Rb | 0.09 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Ru | 0.23 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Cr | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Cu | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Sb | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Sn | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Se | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Ce | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Cs | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Rh | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Te | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Al | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Na | <0.01 | 0.2 M | TBP 0.5 M | HNO3 3.1 M | hydrogenated tetrapropene | 22°C | 15 min | HAR; 0.05M HEDTA | [11] |
| Am241 | >100 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Y | >100 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| La | >100 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Ce(IV) | >100 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Pr | >100 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Nd | >100 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Sm | >100 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Eu | >100 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Eu152 | >100 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Gd | >100 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Zr | >100 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Pd | 4.75 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Ba | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Cd | 0.07 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Mo | 0.24 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Ni | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Sr | 1.23 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Rb | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Ru | 0.21 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Cr | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Cu | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Sb | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Sn | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Se | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Ce | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Cs | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Rh | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Te | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Al | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Na | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR | [11] | |
| Am241 | >100 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [11] | |
| Y | >100 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [11] | |
| La | >100 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [11] | |
| Ce(IV) | >100 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [11] | |
| Pr | >100 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [11] | |
| Nd | >100 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [11] | |
| Sm | >100 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [11] | |
| Eu | 70 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [11] | |
| Eu152 | >100 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [11] | |
| Gd | >100 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [11] | |
| Zr | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [11] | |
| Pd | 0.05 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [11] | |
| Ba | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [11] | |
| Cd | 0.11 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [11] | |
| Mo | 0.19 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [11] | |
| Ni | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [11] | |
| Sr | 3.14 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [11] | |
| Rb | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [11] | |
| Ru | 0.13 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [11] | |
| Cr | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [11] | |
| Cu | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [11] | |
| Sb | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [11] | |
| Sn | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [11] | |
| Se | <0.01 | 0.2 M | HNO3 3.1 M | hydrogenated tetrapropene/1-octanol, 5% | 22°C | 15 min | HAR; 0.05M CDTA | [11] | |
| Eu(III) | 0.097 | 0.1 M | HNO3 1 M | Chloroform | 25°C | 120 min | [11] | ||
| Eu(III) | 300 | 0.1 M | HNO3 1 M | Diethylether | 25°C | 120 min | [11] | ||
| Eu(III) | 0.83 | 0.1 M | HNO3 1 M | Benzene | 25°C | 120 min | [11] | ||
| Eu(III) | 0.79 | 0.1 M | HNO3 1 M | Toluene | 25°C | 120 min | [11] | ||
| Eu(III) | 0.39 | 0.1 M | HNO3 1 M | Tetrachloromethane | 25°C | 120 min | [11] | ||
| Eu(III) | 265 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Eu(III) | >500 | 0.1 M | HNO3 1 M | n-Hexane | 25°C | 120 min | [11] | ||
| Th(IV) | ≈0.158 | 0.1 M | HNO3 0.03 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Th(IV) | ≈0.251 | 0.1 M | HNO3 0.1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Th(IV) | ≈158.5 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| U(VI) | ≈0.003 | 0.1 M | HNO3 0.01 M | n-Dodecane | 25°C | 120 min | [11] | ||
| U(VI) | ≈0.008 | 0.1 M | HNO3 0.1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| U(VI) | ≈0.794 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| U(VI) | ≈6.31 | 0.1 M | HNO3 3 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Np(V) | ≈0.005 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Np(V) | ≈0.126 | 0.1 M | HNO3 3 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Pu(IV) | ≈0.316 | 0.1 M | HNO3 0.35 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Pu(IV) | ≈1.259 | 0.1 M | HNO3 0.12 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Pu(IV) | ≈7.943 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Am(III) | ≈0.005 | 0.1 M | HNO3 0.05 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Am(III) | ≈0.016 | 0.1 M | HNO3 0.1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Am(III) | ≈0.05 | 0.1 M | HNO3 0.2 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Am(III) | ≈0.501 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Am(III) | ≈31.6 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Cm(III) | ≈0.006 | 0.1 M | HNO3 0.01 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Cm(III) | ≈0.04 | 0.1 M | HNO3 0.1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Cm(III) | ≈1.995 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Cm(III) | ≈63.1 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Th(IV) | ≈0.0316 | 0.008 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Th(IV) | ≈0.398 | 0.018 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Th(IV) | ≈7.94 | 0.045 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| U(VI) | ≈0.00316 | 0.018 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| U(VI) | ≈0.0398 | 0.045 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Pu(IV) | ≈0.158 | 0.018 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Pu(IV) | ≈0.501 | 0.045 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Pu(IV) | ≈15.8 | 0.079 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Pu(IV) | ≈63.1 | 0.158 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Pu(IV) | ≈100 | 0.2 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Am(III) | ≈0.00631 | 0.01 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Am(III) | ≈0.0631 | 0.02 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Am(III) | ≈3.16 | 0.05 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Cm(III) | ≈0.00251 | 0.008 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Cm(III) | ≈0.0316 | 0.018 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Cm(III) | ≈1.58 | 0.045 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [11] | ||
| La | ≈0.501 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Ce | ≈0.794 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Pr | ≈1 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Nd | ≈1.78 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Sm | ≈10 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Eu | ≈0.0398 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Gd | ≈20 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Tb | ≈17.8 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Dy | ≈63.1 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Ho | ≈79.4 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Er | ≈141 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Tm | ≈158 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Yb | ≈178 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Lu | ≈178 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Am | ≈200 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Cm | ≈0.562 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Cf | ≈1.58 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 120 min | [11] | ||
| La(III) | 5.3 | 0.1 M | HNO3 1.0 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Ce | ≈5.01 | 0.1 M | HNO3 1.0 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Pr | ≈10 | 0.1 M | HNO3 1.0 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Nd | ≈15.8 | 0.1 M | HNO3 1.0 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Sm | ≈39.8 | 0.1 M | HNO3 1.0 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Eu(III) | 265 | 0.1 M | HNO3 1.0 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Gd | ≈251 | 0.1 M | HNO3 1.0 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Tb | ≈501 | 0.1 M | HNO3 1.0 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Dy | ≈501 | 0.1 M | HNO3 1.0 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Ho | ≈562 | 0.1 M | HNO3 1.0 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Er | ≈631 | 0.1 M | HNO3 1.0 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Tm | ≈631 | 0.1 M | HNO3 1.0 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Yb | ≈631 | 0.1 M | HNO3 1.0 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Lu(III) | 631 | 0.1 M | HNO3 1.0 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Th(IV) | 147 | 0.1 M | HNO3 1.0 M | n-Dodecane | 25°C | 120 min | [11] | ||
| U(VI) | 0.8 | 0.1 M | HNO3 1.0 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Np(V) | 0.0056 | 0.1 M | HNO3 1.0 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Am(III) | 30 | 0.1 M | HNO3 1.0 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Cm(III) | 78 | 0.1 M | HNO3 1.0 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Cf(III) | 156 | 0.1 M | HNO3 1.0 M | n-Dodecane | 25°C | 120 min | [11] | ||
| Am(III) | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [12] |
| Cm(III) | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [12] |
| Cf(III) | >1000 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [12] |
| Eu | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [12] |
| Gd | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [12] |
| Nd | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [12] |
| Pr | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [12] |
| Sm | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [12] |
| Y | >1000 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [12] |
| La | 32 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [12] |
| Ce | 98 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [12] |
| Mo | 0.081 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [12] |
| Pd | 0.16 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [12] |
| Sr | 1.1 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [12] |
| Zr | 0.68 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [12] |
| Ru | 0.17 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [12] |
| Eu(III)152 | ≈1000.00 | 0.2 M | TBP 0.5 M | HNO3 3 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Cm(III)244 | ≈0.25 | 0.2 M | TBP 0.5 M | HNO3 0.1 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Cm(III)244 | ≈1.15 | 0.2 M | TBP 0.5 M | HNO3 0.2 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Cm(III)244 | ≈12.53 | 0.2 M | TBP 0.5 M | HNO3 0.5 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Cm(III)244 | ≈157.06 | 0.2 M | TBP 0.5 M | HNO3 1 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Cm(III)244 | ≈1435.04 | 0.2 M | TBP 0.5 M | HNO3 2 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Cm(III)244 | ≈2253.93 | 0.2 M | TBP 0.5 M | HNO3 3 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Am(III)241 | ≈0.16 | 0.2 M | TBP 0.5 M | HNO3 0.1 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Am(III)241 | ≈0.96 | 0.2 M | TBP 0.5 M | HNO3 0.2 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Am(III)241 | ≈8.73 | 0.2 M | TBP 0.5 M | HNO3 0.5 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Am(III)241 | ≈95.59 | 0.2 M | TBP 0.5 M | HNO3 1 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Am(III)241 | ≈834.77 | 0.2 M | TBP 0.5 M | HNO3 2 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Am(III)241 | ≈1968.42 | 0.2 M | TBP 0.5 M | HNO3 3 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| U(VI) | ≈0.15 | 0.2 M | TBP 0.5 M | HNO3 0.1 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| U(VI) | ≈0.42 | 0.2 M | TBP 0.5 M | HNO3 0.2 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| U(VI) | ≈1.64 | 0.2 M | TBP 0.5 M | HNO3 0.5 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| U(VI) | ≈4.24 | 0.2 M | TBP 0.5 M | HNO3 1 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| U(VI) | ≈11.98 | 0.2 M | TBP 0.5 M | HNO3 2 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| U(VI) | ≈21.54 | 0.2 M | TBP 0.5 M | HNO3 3 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Th(IV) | ≈0.73 | 0.2 M | TBP 0.5 M | HNO3 0.16 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Th(IV) | ≈3.09 | 0.2 M | TBP 0.5 M | HNO3 0.245 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Th(IV) | ≈29.55 | 0.2 M | TBP 0.5 M | HNO3 0.56 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Th(IV) | ≈157.06 | 0.2 M | TBP 0.5 M | HNO3 1.06 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Th(IV) | ≈1000.00 | 0.2 M | TBP 0.5 M | HNO3 2.13 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Th(IV) | ≈2059.33 | 0.2 M | TBP 0.5 M | HNO3 3.12 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Zr | ≈0.01 | 0.2 M | TBP 0.5 M | HNO3 0.09 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Zr | ≈0.08 | 0.2 M | TBP 0.5 M | HNO3 0.18 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Zr | ≈15.51 | 0.2 M | TBP 0.5 M | HNO3 0.5 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Zr | ≈124.52 | 0.2 M | TBP 0.5 M | HNO3 1 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Zr | ≈84.83 | 0.2 M | TBP 0.5 M | HNO3 2 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Zr | ≈182.77 | 0.2 M | TBP 0.5 M | HNO3 3 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Pd | ≈2.83 | 0.2 M | TBP 0.5 M | HNO3 0.09 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Pd | ≈2.04 | 0.2 M | TBP 0.5 M | HNO3 0.18 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Pd | ≈1.73 | 0.2 M | TBP 0.5 M | HNO3 0.5 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Pd | ≈1.64 | 0.2 M | TBP 0.5 M | HNO3 1 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Pd | ≈1.93 | 0.2 M | TBP 0.5 M | HNO3 2 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Pd | ≈1.83 | 0.2 M | TBP 0.5 M | HNO3 3 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Sr | ≈0.01 | 0.2 M | TBP 0.5 M | HNO3 0.09 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Sr | ≈0.01 | 0.2 M | TBP 0.5 M | HNO3 0.18 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Sr | ≈0.06 | 0.2 M | TBP 0.5 M | HNO3 0.5 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Sr | ≈0.39 | 0.2 M | TBP 0.5 M | HNO3 1 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Sr | ≈1.12 | 0.2 M | TBP 0.5 M | HNO3 2 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Sr | ≈1.25 | 0.2 M | TBP 0.5 M | HNO3 3 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Ru | ≈0.01 | 0.2 M | TBP 0.5 M | HNO3 0.09 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Ru | ≈0.02 | 0.2 M | TBP 0.5 M | HNO3 0.18 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Ru | ≈0.08 | 0.2 M | TBP 0.5 M | HNO3 0.5 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Ru | ≈0.12 | 0.2 M | TBP 0.5 M | HNO3 1 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Ru | ≈0.15 | 0.2 M | TBP 0.5 M | HNO3 2 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Ru | ≈0.17 | 0.2 M | TBP 0.5 M | HNO3 3 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Mo | ≈18.28 | 0.2 M | TBP 0.5 M | HNO3 0.09 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Mo | ≈6.45 | 0.2 M | TBP 0.5 M | HNO3 0.18 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Mo | ≈0.24 | 0.2 M | TBP 0.5 M | HNO3 0.5 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Mo | ≈0.03 | 0.2 M | TBP 0.5 M | HNO3 1 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Mo | ≈0.04 | 0.2 M | TBP 0.5 M | HNO3 2 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Mo | ≈0.06 | 0.2 M | TBP 0.5 M | HNO3 3 M | hydrogenated tetrapropene | 22°C | 15 min | [12] | |
| Am(III) | ≈0.03 | 0.1 M | HNO3 0.01 M | hydrogenated tetrapropene | 22°C | 15 min | [13] | ||
| Am(III) | ≈0.09 | 0.1 M | HNO3 0.1 M | hydrogenated tetrapropene | 22°C | 15 min | [13] | ||
| Am(III) | ≈20 | 0.1 M | HNO3 1 M | hydrogenated tetrapropene | 22°C | 15 min | [13] | ||
| Am(III) | ≈190 | 0.1 M | HNO3 2 M | hydrogenated tetrapropene | 22°C | 15 min | [13] | ||
| Am(III) | ≈310 | 0.1 M | HNO3 3 M | hydrogenated tetrapropene | 22°C | 15 min | [13] | ||
| Am(III) | ≈500 | 0.1 M | HNO3 4 M | hydrogenated tetrapropene | 22°C | 15 min | [13] | ||
| Eu(III) | ≈0.08 | 0.1 M | HNO3 0.01 M | hydrogenated tetrapropene | 22°C | 15 min | [13] | ||
| Eu(III) | ≈0.4 | 0.1 M | HNO3 0.1 M | hydrogenated tetrapropene | 22°C | 15 min | [13] | ||
| Eu(III) | ≈90 | 0.1 M | HNO3 1 M | hydrogenated tetrapropene | 22°C | 15 min | [13] | ||
| Eu(III) | ≈200 | 0.1 M | HNO3 2 M | hydrogenated tetrapropene | 22°C | 15 min | [13] | ||
| Eu(III) | ≈310 | 0.1 M | HNO3 3 M | hydrogenated tetrapropene | 22°C | 15 min | [13] | ||
| Eu(III) | ≈500 | 0.1 M | HNO3 4 M | hydrogenated tetrapropene | 22°C | 15 min | [13] | ||
| Am(III) | 6 | 0.05 M | HNO3 1 M | hydrogenated tetrapropene | 21°C | 60 min | 8 MBq/L Am; 8 MBq/L Eu | [14] | |
| Eu(III) | 57 | 0.05 M | HNO3 1 M | hydrogenated tetrapropene | 21°C | 60 min | 8 MBq/L Am; 8 MBq/L Eu | [14] | |
| Am(III) | ≈4.5 | 0.05 M | HNO3 1 M | hydrogenated tetrapropene | 21°C | 60 min | 8 MBq/L Am; 8 MBq/L Eu; Multiple Ln | [14] | |
| Eu(III) | ≈40 | 0.05 M | HNO3 1 M | hydrogenated tetrapropene | 21°C | 60 min | 8 MBq/L Am; 8 MBq/L Eu; Multiple Ln | [14] | |
| La(III) | ≈52 | 0.05 M | HNO3 1 M | hydrogenated tetrapropene | 21°C | 60 min | Multiple Ln | [14] | |
| Ce(III) | ≈72 | 0.05 M | HNO3 1 M | hydrogenated tetrapropene | 21°C | 60 min | Multiple Ln | [14] | |
| Pr(III) | ≈70 | 0.05 M | HNO3 1 M | hydrogenated tetrapropene | 21°C | 60 min | Multiple Ln | [14] | |
| Nd(III) | ≈42 | 0.05 M | HNO3 1 M | hydrogenated tetrapropene | 21°C | 60 min | Multiple Ln | [14] | |
| Sm(III) | ≈114 | 0.05 M | HNO3 1 M | hydrogenated tetrapropene | 21°C | 60 min | Multiple Ln | [14] | |
| Eu(III) | ≈39 | 0.05 M | HNO3 1 M | hydrogenated tetrapropene | 21°C | 60 min | Multiple Ln | [14] | |
| Gd(III) | ≈80 | 0.05 M | HNO3 1 M | hydrogenated tetrapropene | 21°C | 60 min | Multiple Ln | [14] | |
| Y(III) | ≈0.0017 | 0.1 M | n-Dodecane | 25°C | 60 min | [15] | |||
| Y(III) | ≈0.21 | 0.1 M | HNO3 0.1 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Y(III) | ≈110 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Y(III) | ≈250 | 0.1 M | HNO3 2 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Y(III) | ≈280 | 0.1 M | HNO3 3 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Y(III) | ≈190 | 0.1 M | HNO3 4 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Y(III) | ≈55 | 0.1 M | HNO3 6 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Sr(II) | ≈0.00075 | 0.1 M | HNO3 0.1 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Ag | 0.62 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [15] |
| Cd | 0.039 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [15] |
| Ba | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [15] |
| Cr | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [15] |
| Cs | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [15] |
| Cu | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [15] |
| Fe | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [15] |
| Na | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [15] |
| Ni | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [15] |
| Rb | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [15] |
| Rh | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [15] |
| Sb | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [15] |
| Se | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [15] |
| Sn | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [15] |
| Te | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid | [15] |
| Am(III) | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Cm(III) | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Cf(III) | >1000 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Eu | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Gd | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Nd | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Pr | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Sm | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Y | >1000 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| La | 31 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Ce | 108 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Mo | 0.071 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Pd | 0.091 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Sr | 0.84 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Zr | 0.26 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Ru | 0.22 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Ag | 0.28 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Cd | 0.039 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Ba | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Cr | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Cs | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Cu | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Fe | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Na | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Ni | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Rb | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Rh | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Sb | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Se | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Sn | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Te | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.2M Oxalic Acid | [15] |
| Am(III) | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Cm(III) | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Cf(III) | >1000 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Eu | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Gd | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Nd | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Pr | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Sm | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Y | >1000 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| La | 44 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Ce | 170 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Mo | 0.087 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Pd | 0.048 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Sr | 1.5 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Zr | 0.1 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Ru | 0.2 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Ag | 0.12 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Cd | 0.05 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Ba | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Cr | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Cs | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Cu | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Fe | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Na | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Ni | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Rb | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Rh | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Sb | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Se | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Sn | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Te | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.25M Oxalic Acid | [15] |
| Am(III) | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Cm(III) | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Cf(III) | >1000 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Eu | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Gd | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Nd | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Pr | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Sm | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Y | >1000 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| La | 43 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Ce | 164 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Mo | 0.11 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Pd | 0.055 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Sr | 1.6 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Zr | 0.097 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Ru | 0.19 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Ag | 0.11 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Cd | 0.052 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Ba | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Cr | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Cs | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Cu | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Fe | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Na | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Ni | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Rb | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Rh | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Sb | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Se | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Sn | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Te | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.3M Oxalic Acid | [15] |
| Am(III) | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Cm(III) | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Cf(III) | >1000 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Eu | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Gd | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Nd | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Pr | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Sm | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Y | >1000 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| La | 43 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Ce | 164 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Mo | 0.12 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Pd | 0.046 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Sr | 1.8 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Zr | 0.056 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Ru | 0.16 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Ag | 0.066 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Cd | 0.059 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Ba | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Cr | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Cs | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Cu | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Fe | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Na | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Ni | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Rb | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Rh | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Sb | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Se | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Sn | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Te | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.35M Oxalic Acid | [15] |
| Am(III) | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Cm(III) | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Cf(III) | >1000 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Eu | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Gd | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Nd | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Pr | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Sm | >200 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Y | >1000 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| La | 51 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Ce | 197 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Mo | 0.14 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Pd | 0.038 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Sr | 1.9 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Zr | 0.042 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Ru | 0.18 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Ag | 0.056 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Cd | 0.062 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Ba | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Cr | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Cs | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Cu | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Fe | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Na | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Ni | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Rb | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Rh | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Sb | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Se | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Sn | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Te | <0.01 | 0.2 M | HEDTA 0.05 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.4M Oxalic Acid | [15] |
| Am(III) | >200 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.05M Oxalic Acid; 0.05M HEDTA | [15] |
| Cm(III) | >200 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.05M Oxalic Acid; 0.05M HEDTA | [15] |
| Cf(III) | >200 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.05M Oxalic Acid; 0.05M HEDTA | [15] |
| Ln(III) | >200 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.05M Oxalic Acid; 0.05M HEDTA | [15] |
| Mo | 0.06 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.05M Oxalic Acid; 0.05M HEDTA | [15] |
| Pd | 0.3 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.05M Oxalic Acid; 0.05M HEDTA | [15] |
| Ru | 0.23 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.05M Oxalic Acid; 0.05M HEDTA | [15] |
| Sr | 0.4 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.05M Oxalic Acid; 0.05M HEDTA | [15] |
| Zr | 5.9 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.05M Oxalic Acid; 0.05M HEDTA | [15] |
| F.P. | <0.03 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.05M Oxalic Acid; 0.05M HEDTA | [15] |
| Am(III) | >200 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.05M Oxalic Acid; 0.05M HEDTA | [15] |
| Cm(III) | >200 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.05M Oxalic Acid; 0.05M HEDTA | [15] |
| Cf(III) | >200 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.05M Oxalic Acid; 0.05M HEDTA | [15] |
| Ln(III) | >200 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.05M Oxalic Acid; 0.05M HEDTA | [15] |
| Mo | 0.08 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.05M Oxalic Acid; 0.05M HEDTA | [15] |
| Pd | 0.36 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.05M Oxalic Acid; 0.05M HEDTA | [15] |
| Ru | 0.22 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.05M Oxalic Acid; 0.05M HEDTA | [15] |
| Sr | 0.59 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.05M Oxalic Acid; 0.05M HEDTA | [15] |
| Zr | 5.8 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.05M Oxalic Acid; 0.05M HEDTA | [15] |
| F.P. | <0.03 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.05M Oxalic Acid; 0.05M HEDTA | [15] |
| Am(III) | >200 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.10M Oxalic Acid; 0.05M HEDTA | [15] |
| Cm(III) | >200 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.10M Oxalic Acid; 0.05M HEDTA | [15] |
| Cf(III) | >200 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.10M Oxalic Acid; 0.05M HEDTA | [15] |
| Ln(III) | >200 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.10M Oxalic Acid; 0.05M HEDTA | [15] |
| Mo | 0.05 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.10M Oxalic Acid; 0.05M HEDTA | [15] |
| Pd | 0.16 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.10M Oxalic Acid; 0.05M HEDTA | [15] |
| Ru | 0.22 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.10M Oxalic Acid; 0.05M HEDTA | [15] |
| Sr | 0.54 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.10M Oxalic Acid; 0.05M HEDTA | [15] |
| Zr | 0.82 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.10M Oxalic Acid; 0.05M HEDTA | [15] |
| F.P. | <0.03 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.10M Oxalic Acid; 0.05M HEDTA | [15] |
| Am(III) | >200 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.10M Oxalic Acid; 0.05M HEDTA | [15] |
| Cm(III) | >200 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.10M Oxalic Acid; 0.05M HEDTA | [15] |
| Cf(III) | >200 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.10M Oxalic Acid; 0.05M HEDTA | [15] |
| Ln(III) | >200 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.10M Oxalic Acid; 0.05M HEDTA | [15] |
| Mo | 0.06 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.10M Oxalic Acid; 0.05M HEDTA | [15] |
| Pd | 0.18 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.10M Oxalic Acid; 0.05M HEDTA | [15] |
| Ru | 0.19 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.10M Oxalic Acid; 0.05M HEDTA | [15] |
| Sr | 0.73 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.10M Oxalic Acid; 0.05M HEDTA | [15] |
| Zr | 1.1 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.10M Oxalic Acid; 0.05M HEDTA | [15] |
| F.P. | <0.03 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.10M Oxalic Acid; 0.05M HEDTA | [15] |
| Am(III) | >200 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid; 0.05M HEDTA | [15] |
| Cm(III) | >200 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid; 0.05M HEDTA | [15] |
| Cf(III) | >200 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid; 0.05M HEDTA | [15] |
| Ln(III) | >200 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid; 0.05M HEDTA | [15] |
| Mo | 0.04 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid; 0.05M HEDTA | [15] |
| Pd | 0.07 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid; 0.05M HEDTA | [15] |
| Ru | 0.25 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid; 0.05M HEDTA | [15] |
| Sr | 0.44 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid; 0.05M HEDTA | [15] |
| Zr | 0.18 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid; 0.05M HEDTA | [15] |
| F.P. | <0.03 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid; 0.05M HEDTA | [15] |
| Am(III) | >200 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid; 0.05M HEDTA | [15] |
| Cm(III) | >200 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid; 0.05M HEDTA | [15] |
| Cf(III) | >200 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid; 0.05M HEDTA | [15] |
| Ln(III) | >200 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid; 0.05M HEDTA | [15] |
| Mo | 0.05 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid; 0.05M HEDTA | [15] |
| Pd | 0.07 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid; 0.05M HEDTA | [15] |
| Ru | 0.2 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid; 0.05M HEDTA | [15] |
| Sr | 0.77 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid; 0.05M HEDTA | [15] |
| Zr | 0.19 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid; 0.05M HEDTA | [15] |
| F.P. | <0.03 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.15M Oxalic Acid; 0.05M HEDTA | [15] |
| Am(III) | >200 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.20M Oxalic Acid; 0.05M HEDTA | [15] |
| Cm(III) | >200 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.20M Oxalic Acid; 0.05M HEDTA | [15] |
| Cf(III) | >200 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.20M Oxalic Acid; 0.05M HEDTA | [15] |
| Ln(III) | >200 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.20M Oxalic Acid; 0.05M HEDTA | [15] |
| Mo | 0.04 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.20M Oxalic Acid; 0.05M HEDTA | [15] |
| Pd | 0.03 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.20M Oxalic Acid; 0.05M HEDTA | [15] |
| Ru | 0.25 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.20M Oxalic Acid; 0.05M HEDTA | [15] |
| Sr | 0.42 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.20M Oxalic Acid; 0.05M HEDTA | [15] |
| Zr | 0.06 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.20M Oxalic Acid; 0.05M HEDTA | [15] |
| F.P. | <0.03 | 0.2 M | TBP 0.5 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.20M Oxalic Acid; 0.05M HEDTA | [15] |
| Am(III) | >200 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.20M Oxalic Acid; 0.05M HEDTA | [15] |
| Cm(III) | >200 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.20M Oxalic Acid; 0.05M HEDTA | [15] |
| Cf(III) | >200 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.20M Oxalic Acid; 0.05M HEDTA | [15] |
| Ln(III) | >200 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.20M Oxalic Acid; 0.05M HEDTA | [15] |
| Mo | 0.05 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.20M Oxalic Acid; 0.05M HEDTA | [15] |
| Pd | 0.04 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.20M Oxalic Acid; 0.05M HEDTA | [15] |
| Ru | 0.21 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.20M Oxalic Acid; 0.05M HEDTA | [15] |
| Sr | 0.64 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.20M Oxalic Acid; 0.05M HEDTA | [15] |
| Zr | 0.09 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.20M Oxalic Acid; 0.05M HEDTA | [15] |
| F.P. | <0.03 | 0.2 M | TBP 0.25 M | HNO3 3.2 M | hydrogenated tetrapropene | 22°C | 15 min | Synthetic Highly Active Raffinate; 0.20M Oxalic Acid; 0.05M HEDTA | [15] |
| Cf(III)252 | ≈2.25 | 0.2 M | TBP 0.5 M | HNO3 0.1 M | hydrogenated tetrapropene | 22°C | 15 min | [15] | |
| Cf(III)252 | ≈13.11 | 0.2 M | TBP 0.5 M | HNO3 0.2 M | hydrogenated tetrapropene | 22°C | 15 min | [15] | |
| Cf(III)252 | ≈188.15 | 0.2 M | TBP 0.5 M | HNO3 0.5 M | hydrogenated tetrapropene | 22°C | 15 min | [15] | |
| Cf(III)252 | ≈1371.69 | 0.2 M | TBP 0.5 M | HNO3 1 M | hydrogenated tetrapropene | 22°C | 15 min | [15] | |
| Cf(III)252 | ≈5080.22 | 0.2 M | TBP 0.5 M | HNO3 2 M | hydrogenated tetrapropene | 22°C | 15 min | [15] | |
| Cf(III)252 | ≈3540.13 | 0.2 M | TBP 0.5 M | HNO3 3 M | hydrogenated tetrapropene | 22°C | 15 min | [15] | |
| Eu(III)152 | ≈1.00 | 0.2 M | TBP 0.5 M | HNO3 0.1 M | hydrogenated tetrapropene | 22°C | 15 min | [15] | |
| Eu(III)152 | ≈5.08 | 0.2 M | TBP 0.5 M | HNO3 0.2 M | hydrogenated tetrapropene | 22°C | 15 min | [15] | |
| Eu(III)152 | ≈55.60 | 0.2 M | TBP 0.5 M | HNO3 0.5 M | hydrogenated tetrapropene | 22°C | 15 min | [15] | |
| Eu(III)152 | ≈424.08 | 0.2 M | TBP 0.5 M | HNO3 1 M | hydrogenated tetrapropene | 22°C | 15 min | [15] | |
| Eu(III)152 | ≈1000.00 | 0.2 M | TBP 0.5 M | HNO3 2 M | hydrogenated tetrapropene | 22°C | 15 min | [15] | |
| Y(III) | ≈2500 | 0.2 M | HNO3 0.5 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Y(III) | ≈5000 | 0.2 M | HNO3 0.75 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Y(III) | ≈17000 | 0.2 M | HNO3 1 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Dy(III) | ≈280 | 0.2 M | HNO3 0.15 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Dy(III) | ≈700 | 0.2 M | HNO3 0.25 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Dy(III) | ≈1500 | 0.2 M | HNO3 0.35 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Dy(III) | ≈4800 | 0.2 M | HNO3 0.5 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Dy(III) | ≈7000 | 0.2 M | HNO3 0.75 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Tb(III) | ≈200 | 0.2 M | HNO3 0.15 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Tb(III) | ≈500 | 0.2 M | HNO3 0.25 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Tb(III) | ≈900 | 0.2 M | HNO3 0.35 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Tb(III) | ≈2200 | 0.2 M | HNO3 0.5 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Tb(III) | ≈7000 | 0.2 M | HNO3 0.75 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Tb(III) | ≈13000 | 0.2 M | HNO3 1 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Gd(III) | ≈80 | 0.2 M | HNO3 0.15 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Gd(III) | ≈200 | 0.2 M | HNO3 0.25 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Gd(III) | ≈400 | 0.2 M | HNO3 0.35 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Gd(III) | ≈600 | 0.2 M | HNO3 0.5 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Gd(III) | ≈2800 | 0.2 M | HNO3 0.75 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Gd(III) | ≈4800 | 0.2 M | HNO3 1 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Eu(III) | ≈80 | 0.2 M | HNO3 0.15 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Eu(III) | ≈200 | 0.2 M | HNO3 0.25 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Eu(III) | ≈400 | 0.2 M | HNO3 0.35 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Eu(III) | ≈1000 | 0.2 M | HNO3 0.5 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Eu(III) | ≈2800 | 0.2 M | HNO3 0.75 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Eu(III) | ≈8500 | 0.2 M | HNO3 1 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Sm(III) | ≈40 | 0.2 M | HNO3 0.15 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Sm(III) | ≈100 | 0.2 M | HNO3 0.25 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Sm(III) | ≈270 | 0.2 M | HNO3 0.35 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Sm(III) | ≈470 | 0.2 M | HNO3 0.5 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Sm(III) | ≈1200 | 0.2 M | HNO3 0.75 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Sm(III) | ≈2800 | 0.2 M | HNO3 1 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Cm(III) | ≈18 | 0.2 M | HNO3 0.15 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Cm(III) | ≈40 | 0.2 M | HNO3 0.25 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Cm(III) | ≈75 | 0.2 M | HNO3 0.35 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Cm(III) | ≈170 | 0.2 M | HNO3 0.5 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Am(III) | ≈11 | 0.2 M | HNO3 0.15 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Am(III) | ≈25 | 0.2 M | HNO3 0.25 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Am(III) | ≈50 | 0.2 M | HNO3 0.35 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Am(III) | ≈100 | 0.2 M | HNO3 0.5 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Am(III) | ≈300 | 0.2 M | HNO3 0.75 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Am(III) | ≈600 | 0.2 M | HNO3 1 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Nd(III) | ≈6 | 0.2 M | HNO3 0.15 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Nd(III) | ≈17 | 0.2 M | HNO3 0.25 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Nd(III) | ≈30 | 0.2 M | HNO3 0.35 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Nd(III) | ≈70 | 0.2 M | HNO3 0.5 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Nd(III) | ≈210 | 0.2 M | HNO3 0.75 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Nd(III) | ≈450 | 0.2 M | HNO3 1 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Pr(III) | ≈3 | 0.2 M | HNO3 0.15 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Pr(III) | ≈8 | 0.2 M | HNO3 0.25 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Pr(III) | ≈12 | 0.2 M | HNO3 0.35 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Pr(III) | ≈30 | 0.2 M | HNO3 0.5 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Pr(III) | ≈90 | 0.2 M | HNO3 0.75 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Pr(III) | ≈190 | 0.2 M | HNO3 1 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Ce(III) | ≈1.9 | 0.2 M | HNO3 0.15 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Ce(III) | ≈4.2 | 0.2 M | HNO3 0.25 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Ce(III) | ≈7.5 | 0.2 M | HNO3 0.35 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Ce(III) | ≈17 | 0.2 M | HNO3 0.5 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Ce(III) | ≈45 | 0.2 M | HNO3 0.75 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Ce(III) | ≈85 | 0.2 M | HNO3 1 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| La(III) | ≈1 | 0.2 M | HNO3 0.15 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| La(III) | ≈2.5 | 0.2 M | HNO3 0.25 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| La(III) | ≈4 | 0.2 M | HNO3 0.35 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| La(III) | ≈9 | 0.2 M | HNO3 0.5 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| La(III) | ≈21 | 0.2 M | HNO3 0.75 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| La(III) | ≈38 | 0.2 M | HNO3 1 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | [15] | ||
| Am(III) | 23 | 0.2 M | HEDTA 0.02 M | HNO3 4.2 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | 0.2M Oxalic Acid | [15] |
| Eu(III) | 180 | 0.2 M | HEDTA 0.02 M | HNO3 4.2 M | hydrogenated tetrapropene/1-octanol, 5% | 20°C | 15 min | 0.2M Oxalic Acid | [15] |
| Am(III) | 220 | 0.1 M | HNO3 1 M | Nitrobenzene | 25°C | 120 min | [15] | ||
| Am(III) | 9.9 | 0.1 M | HNO3 1 M | 1,2-Dichloroethane | 25°C | 120 min | [15] | ||
| Am(III) | 81 | 0.1 M | HNO3 1 M | 1-Octanol | 25°C | 120 min | [15] | ||
| Am(III) | >500 | 0.1 M | HNO3 1 M | Ethyl Acetate | 25°C | 120 min | [15] | ||
| Am(III) | 0.12 | 0.1 M | HNO3 1 M | Chloroform | 25°C | 120 min | [15] | ||
| Am(III) | 100 | 0.1 M | HNO3 1 M | Diethylether | 25°C | 120 min | [15] | ||
| Am(III) | 0.39 | 0.1 M | HNO3 1 M | Benzene | 25°C | 120 min | [15] | ||
| Am(III) | 0.3 | 0.1 M | HNO3 1 M | Toluene | 25°C | 120 min | [15] | ||
| Am(III) | 0.095 | 0.1 M | HNO3 1 M | Tetrachloromethane | 25°C | 120 min | [15] | ||
| Am(III) | 30 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 120 min | [15] | ||
| Am(III) | 33 | 0.1 M | HNO3 1 M | n-Hexane | 25°C | 120 min | [15] | ||
| Eu(III) | >500 | 0.1 M | HNO3 1 M | Nitrobenzene | 25°C | 120 min | [15] | ||
| Eu(III) | 34 | 0.1 M | HNO3 1 M | 1,2-Dichloroethane | 25°C | 120 min | [15] | ||
| Eu(III) | >500 | 0.1 M | HNO3 1 M | 1-Octanol | 25°C | 120 min | [15] | ||
| Eu(III) | >500 | 0.1 M | HNO3 1 M | Ethyl Acetate | 25°C | 120 min | [15] | ||
| Sr(II) | ≈0.11 | 0.1 M | HNO3 1 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Sr(II) | ≈0.6 | 0.1 M | HNO3 2 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Sr(II) | ≈1.1 | 0.1 M | HNO3 3 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Sr(II) | ≈1.2 | 0.1 M | HNO3 4 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Sr(II) | ≈0.05 | 0.1 M | HNO3 6 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Y(III) | ≈0.0003 | 0.1 M | n-Dodecane | 25°C | 60 min | [15] | |||
| Y(III) | ≈0.00035 | 0.1 M | HCl 0.1 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Y(III) | ≈0.0012 | 0.1 M | HCl 1 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Y(III) | ≈0.025 | 0.1 M | HCl 2 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Y(III) | ≈0.45 | 0.1 M | HCl 3 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Y(III) | ≈140 | 0.1 M | HCl 4 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Y(III) | ≈900 | 0.1 M | HCl 6 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Sr(II) | ≈0.0008 | 0.1 M | n-Dodecane | 25°C | 60 min | [15] | |||
| Sr(II) | ≈0.0005 | 0.1 M | HCl 0.1 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Sr(II) | ≈0.0048 | 0.1 M | HCl 1 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Sr(II) | ≈0.0048 | 0.1 M | HCl 2 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Sr(II) | ≈0.0034 | 0.1 M | HCl 3 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Sr(II) | ≈0.0055 | 0.1 M | HCl 4 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Sr(II) | ≈0.017 | 0.1 M | HCl 6 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Y(III) | ≈0.191 | 0.001 M | HCl 6 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Y(III) | ≈1 | 0.002 M | HCl 6 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Y(III) | ≈4.22 | 0.003 M | HCl 6 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Y(III) | ≈10.7 | 0.004 M | HCl 6 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Y(III) | ≈24 | 0.005 M | HCl 6 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Y(III) | ≈0 | 0.1 M | HCl 6 M | n-Dodecane | 25°C | 0 min | [15] | ||
| Y(III) | ≈71 | 0.1 M | HCl 6 M | n-Dodecane | 25°C | 5 min | [15] | ||
| Y(III) | ≈72 | 0.1 M | HCl 6 M | n-Dodecane | 25°C | 10 min | [15] | ||
| Y(III) | ≈82 | 0.1 M | HCl 6 M | n-Dodecane | 25°C | 30 min | [15] | ||
| Y(III) | ≈66 | 0.1 M | HCl 6 M | n-Dodecane | 25°C | 60 min | [15] | ||
| Y(III) | ≈72 | 0.1 M | HCl 6 M | n-Dodecane | 25°C | 120 min | [15] | ||
| Y(III) | ≈7 | 0.005 M | HNO3 6 M | n-Dodecane | 10°C | 60 min | [15] | ||
| Y(III) | ≈12 | 0.005 M | HNO3 6 M | n-Dodecane | 20°C | 60 min | [15] | ||
| Y(III) | ≈20 | 0.005 M | HNO3 6 M | n-Dodecane | 30°C | 0 min | [15] | ||
| Y(III) | ≈55 | 0.005 M | HNO3 6 M | n-Dodecane | 40°C | 5 min | [15] | ||
| Y(III) | ≈0.015 | 0.001 M | HCl 6 M | n-Dodecane | 10°C | 10 min | [15] | ||
| Y(III) | ≈0.05 | 0.001 M | HCl 6 M | n-Dodecane | 20°C | 30 min | [15] | ||
| Y(III) | ≈0.1 | 0.001 M | HCl 6 M | n-Dodecane | 30°C | 60 min | [15] | ||
| Y(III) | ≈0.17 | 0.001 M | HCl 6 M | n-Dodecane | 40°C | 120 min | [15] | ||
| Am(III) | 170 | 0.1 M | HNO3 3 M | HPT/1-Octanol, 1:1 | 22°C | 10 min | [15] | ||
| Eu(III) | 330 | 0.1 M | HNO3 3 M | HPT/1-Octanol, 1:1 | 22°C | 10 min | [15] | ||
| La | ≈0.50 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 30 min | [16] | ||
| Ce | ≈0.80 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 30 min | [16] | ||
| Pr | ≈1.12 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 30 min | [16] | ||
| Nd | ≈1.86 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 30 min | [16] | ||
| Sm | ≈9.62 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 30 min | [16] | ||
| Eu | ≈18.94 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 30 min | [16] | ||
| Gd | ≈18.04 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 30 min | [16] | ||
| Tb | ≈59.02 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 30 min | [16] | ||
| Dy | ≈78.90 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 30 min | [16] | ||
| Ho | ≈144.43 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 30 min | [16] | ||
| Er | ≈155.30 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 30 min | [16] | ||
| Tm | ≈179.56 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 30 min | [16] | ||
| Yb | ≈183.95 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 30 min | [16] | ||
| Lu | ≈207.60 | 0.1 M | HNO3 0.5 M | n-Dodecane | 25°C | 30 min | [16] |
Decontamination factors
| Element | Value | Conc. | Add. ligand | Aq. acid | Organic diluent | Temp. | Additional | Stages | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Na | 1 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [17] | ||
| Rb | 1.01 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate | 16 | [18] | |
| Cs | 0.99 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate | 16 | [18] | |
| Sr | 0.96 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate | 16 | [18] | |
| Ba | 1 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate | 16 | [18] | |
| Zr | 0.86 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate | 16 | [18] | |
| Mo | 0.98 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate | 16 | [18] | |
| Ru | 1.12 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate | 16 | [18] | |
| Rh | 1 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate | 16 | [18] | |
| Pd | 1.05 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate | 16 | [18] | |
| Ag | 0.97 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate | 16 | [18] | |
| Cd | 1 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate | 16 | [18] | |
| Te | 0.94 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate | 16 | [18] | |
| Y | 4042 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate | 16 | [18] | |
| La | >10000 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate | 16 | [18] | |
| Ce | 7336 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate | 16 | [18] | |
| Pr | >10000 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate | 16 | [18] | |
| Nd | >10000 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate | 16 | [18] | |
| Sm | 699 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate | 16 | [18] | |
| Eu | 175 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate | 16 | [18] | |
| Gd | >10000 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate | 16 | [18] | |
| U | 69 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate | 16 | [18] | |
| Am241 | >10000 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate; DFs according to gamma-decay | 16 | [18] | |
| Eu152 | >10000 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate; DFs according to gamma-decay | 16 | [18] | |
| Cs134 | 0.95 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate; DFs according to gamma-decay | 16 | [18] | |
| Cm244 | >10000 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate; DFs according to alpha-decay | 16 | [18] | |
| Cf252 | >10000 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate; DFs according to alpha-decay | 16 | [18] | |
| Am241 | >10000 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; Simulated PUREX Feed; raffinate; DFs according to alpha-decay | 16 | [18] | |
| Rb | 1 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; 60 GWd/tM UO2 fuel | 16 | [19] | |
| Sr | 1 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; 60 GWd/tM UO2 fuel | 16 | [19] | |
| Y | 1600 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; 60 GWd/tM UO2 fuel | 16 | [19] | |
| Zr | 1 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; 60 GWd/tM UO2 fuel | 16 | [19] | |
| Mo | 1 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; 60 GWd/tM UO2 fuel | 16 | [19] | |
| Ru | 1.2 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; 60 GWd/tM UO2 fuel | 16 | [19] | |
| Rh | 1 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; 60 GWd/tM UO2 fuel | 16 | [19] | |
| Ag | 1 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; 60 GWd/tM UO2 fuel | 16 | [19] | |
| Pd | 1 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; 60 GWd/tM UO2 fuel | 16 | [19] | |
| Te | 1 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; 60 GWd/tM UO2 fuel | 16 | [19] | |
| Cs | 1 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; 60 GWd/tM UO2 fuel | 16 | [19] | |
| Ba | 1 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; 60 GWd/tM UO2 fuel | 16 | [19] | |
| La | >1000 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; 60 GWd/tM UO2 fuel | 16 | [19] | |
| Ce | 5400 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; 60 GWd/tM UO2 fuel | 16 | [19] | |
| Pr | 12000 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; 60 GWd/tM UO2 fuel | 16 | [19] | |
| Nd | 15000 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; 60 GWd/tM UO2 fuel | 16 | [19] | |
| Sm | 1600 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; 60 GWd/tM UO2 fuel | 16 | [19] | |
| Eu | >1000 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; 60 GWd/tM UO2 fuel | 16 | [19] | |
| Am | 41000 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; 60 GWd/tM UO2 fuel | 16 | [19] | |
| Cm | 40000 | 0.2 M | TBP 0.5 M | HNO3 4.4 M | hydrogenated tetrapropene | 0.2M Oxalic Acid; 0.05M HEDTA; 60 GWd/tM UO2 fuel | 16 | [19] | |
| Y | 1 | 0.015 M | DMDOHEMA 0.25 M | HNO3 2 M | n-Octanol | TODGA Process Product | 16 | [20] | |
| La | 1 | 0.015 M | DMDOHEMA 0.25 M | HNO3 2 M | n-Octanol | TODGA Process Product | 16 | [20] | |
| Ce | 1 | 0.015 M | DMDOHEMA 0.25 M | HNO3 2 M | n-Octanol | TODGA Process Product | 16 | [20] | |
| Eu152 | 0.98 | 0.05 M | HNO3 3 M | n-dodecane/iso-decanol, 5% | SHLW of PHWR; 0.1M oxalic acid; 0.05M HEDTA | 12 | [21] | ||
| Sr85+Sr89 | 2990 | 0.05 M | HNO3 3 M | n-dodecane/iso-decanol, 5% | SHLW of PHWR; 0.1M oxalic acid; 0.05M HEDTA | 12 | [21] | ||
| Fe59 | 1997 | 0.05 M | HNO3 3 M | n-dodecane/iso-decanol, 5% | SHLW of PHWR; 0.1M oxalic acid; 0.05M HEDTA | 12 | [21] | ||
| Cs137 | 3918 | 0.05 M | HNO3 3 M | n-dodecane/iso-decanol, 5% | SHLW of PHWR; 0.1M oxalic acid; 0.05M HEDTA | 12 | [21] | ||
| Ru106 | 1407 | 0.05 M | HNO3 3 M | n-dodecane/iso-decanol, 5% | SHLW of PHWR; 0.1M oxalic acid; 0.05M HEDTA | 12 | [21] | ||
| Mo99 | 3250 | 0.05 M | HNO3 3 M | n-dodecane/iso-decanol, 5% | SHLW of PHWR; 0.1M oxalic acid; 0.05M HEDTA | 12 | [21] | ||
| Pd109 | 1185 | 0.05 M | HNO3 3 M | n-dodecane/iso-decanol, 5% | SHLW of PHWR; 0.1M oxalic acid; 0.05M HEDTA | 12 | [21] | ||
| Zr95 | 1150 | 0.05 M | HNO3 3 M | n-dodecane/iso-decanol, 5% | SHLW of PHWR; 0.1M oxalic acid; 0.05M HEDTA | 12 | [21] | ||
| Am241 | 2600 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Cm244 | 750 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Eu152 | 1100 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| La | 23000 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Ce | 570000 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Pr | 220000 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Nd | 39000 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Sm | 3400 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Eu | 380 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Gd | 49000 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Y | 47000 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Ru | 1.2 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Pd | 1 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Zr | 1 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Mo | 1 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Sr | 1 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Rh | 1 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Rb | 1 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Ba | 1 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Cs | 1 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Te | 1 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Cd | 1 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Sn | 1 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Sb | 1 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Cu | 1 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Ni | 1 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Cr | 1 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] | ||
| Fe | 1 | 0.2 M | HNO3 4.45 M | hydrogenated tetrapropene/ 1-octanol, 5% | 0.05M CDTA | 16 | [21] |
Solubility
| Solvent | Solubility | Temperature | Ref. |
|---|---|---|---|
| H2O | 0.042 mM | [22] |
References
-
doi:10.1081/SEI-200037727
-
doi:10.1081/SEI-120024552
-
doi:10.15261/serdj.18.93
-
doi:10.1080/07366299.2012.671111
-
doi:10.1081/SEI-100001376
-
doi:10.1080/07366290802672212
-
10.1080/07366299.2012.700591
-
10.1016/j.proche.2012.10.049
-
10.1081/SEI-200066296
-
10.1016/j.hydromet.2014.12.019
-
https://core.ac.uk/download/pdf/82226816.pdf
-
doi:10.1080/07366290701634578
-
doi:10.1080/10610278.2010.506553
-
doi:10.1080/07366299.2012.757160
-
doi:10.1016/j.apradiso.2010.09.016
-
doi:10.1080/07366299.2013.836422
-
doi:10.1080/07366299.2014.952532
-
doi:10.1080/07366290701784175
-
doi:10.1080/07366290802544726
-
doi:10.1080/07366290802672204
-
doi:10.1080/07366299.2011.609392
-
doi:10.1016/j.radphyschem.2006.05.008